Download PDF:
Whole Body Cryotherapy- Benefits for Multiple Sclerosis
Evaluation of serum oxidant/antioxidant balance in multiple sclerosis
Abdullah Acar • M. Ugur Cevik • Osman Evliyaoglu • Ertugrul Uzar • Yusuf Tamam • Adalet Arıkanoglu • Yavuz Yucel • Sefer Varol • Hakan Onder • Nebahat Tasdemir
Received: 1 October 2011 / Accepted: 2 December 2011 Belgian Neurological Society 2012
Abstract
The total oxidative status (TOS)/total antioxidative status (TAS) ratio can provide information on an individual’s absolute oxidative stress index (OSI). We investigated the alterations in the oxidant–antioxidant balance by measuring the oxidant parameters OSI, TOS, and malondialdehyde (MDA) together with the antioxidant parameters such as TAS, and superoxide dismutase (SOD) in patients with relapsing remitting multiple sclerosis (MS). To our knowledge, this is the first study to evaluate OSI in patients with relapsing remitting MS. 35 ambulatory patients with relapsing–remitting MS (35.8 ± 8.7 years) and 32 age- and activity-matched healthy control subjects (35.1 ± 3.7 years) that participated in the study. Serum TAS and TOS levels were determined using new automated methods. MS patients had higher concentrations of MDA (151.5 ± 51.1 vs. 111.3 ± 27.4 nmol/g protein, respectively; p\0.001), TOS (148.1 ± 162.5 vs. 48.3 ± 46.4 mmol H2O2 Equiv./g protein, respectively; p = 0.002), OSI (21124 ± 32543 vs. 5294 ± 5562, respectively; p = 0.008), and SOD (4.5 ± 0.7 vs. 3.4 ± 0.6 U/L, respectively; p\0.001) compared with healthy controls. On the other hand, MS patients had lower concentrations of NO (12.3 ± 6.9 vs. 17.4 ± 2.5 lmol/g protein, respectively; p\0.001) and TAS (0.82 ± 0.27 vs. 0.26 ± 0.15, respectively; p = 0.011) compared with healthy controls. In conclusion, these findings indicate that the oxidative stress plays an important role in the pathogenesis of MS.
Keywords Multiple sclerosis – Pathogenesis – Oxidative stress index – Total oxidant status – Total antioxidant status.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) leading to focal plaques of primary demyelination with a variable degree of axonal and neuronal degeneration. MS is considered a multifactorial disease and MS patients may suffer from a number of clinical symptoms including changes in sensation, visual problems, and muscle weakness together with coordination and speech problems. Although the etiology of MS has not been fully elucidated yet, it is believed that immunological mechanisms are the most important factors in disease initiation and progression [1]. Although different mechanisms may contribute to the demyelination and neurodegeneration in MS, it became clear in recent years that oxidative stress plays the greatest role in the process. The generation of free radicals and a subsequent increase in oxidative stress is a by-product of an inflammatory environment [2, 3]. Increased reactive oxygen species occurring in MS lead to a disruption of membrane integrity by interacting with the lipids, proteins, and nucleic acids. There are several enzymatic and nonenzymatic antioxidant defense systems acting against the harmful effects of these reactive oxygen species. Under normal circumstances, there is a balance between the oxidants and antioxidants. However, excessive production of the reactive oxygen species or decreased antioxidant activity causes an oxidative stress [4, 5]. Accumulated data show that this oxidative stress plays an important role in the pathogenesis of MS. Reactive oxygen species (ROS), generated in excess primarily by the macrophages and leading to oxidative stress, have been suggested as mediators of the demyelination and axonal injury in MS. Additionally, due to the vulnerability of the CNS to the effects of the ROS, depleted cellular antioxidant defense systems in the CNS may increase the injury observed in MS [3]. Previous studies have suggested that oxidative stress and lipid peroxidation play a role in the inflammatory processes and in the pathogenesis of MS [5]. Malondyaldehyde (MDA) is the breakdown product of the most important chain reactions leading to the oxidation of polyunsaturated fatty acids and therefore serves as a reliable oxidant marker of oxidative stress-mediated lipid peroxidation [6]. Serum concentrations of different oxidant species can be measured separately in laboratories; however, these measurements are time consuming, overly expensive, and require complicated techniques. In recent years, total levels of different oxidant species were monitored by determining the total oxidant status (TOS) [7]. Moreover, total antioxidant status (TAS) is a useful estimate of the activity of the total antioxidants in a medium [8]. Consequently, measurements of TAS and TOS can provide information on an individual’s absolute serum Oxidative Stress Index (OSI) which may also include those oxidants and antioxidants not yet known or not easily measured [9]. We investigated the alterations in the oxidant–antioxidant balance by measuring the oxidant parameters OSI, TOS, and MDA together with the antioxidant parameters such as TAS and superoxide dismutase (SOD) in patients with relapsing remitting MS. To our knowledge, this is the first study to evaluate OSI in patients with relapsing remitting MS.
Materials and methods
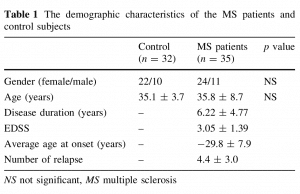
Subjects This study was conducted in the Neurology clinic of Dicle University. 35 ambulatory [Expanded Disability Status Score (EDSS) 0.5–6.5] patients with relapsing–remitting MS (35.8 ± 8.7 years) and 32 age- and activity-matched healthy control subjects (35.1 ± 3.7 years) participated in the study. All the procedures were approved by the Dicle University, Faculty of Medicine Ethics Committee. All study participants signed written informed consent forms before the inclusion in the study. Venous blood samples were obtained between 8–12 a.m. through an intravenous cannula placed in the forearm of the 32 healthy control subjects and 35 patients with definite MS. All patients had relapsing-remitting MS. Exclusion criteria included a relapse, corticosteroid administration within the past 3 months, the presence of infections, other systemic diseases, and pregnancy. None of the patients or control subjects followed any special diet. 32 patients were using interferon beta. Three patients were under treatment with glatiramer acetate. No immunosuppressive therapies were used.
Biochemical analyses
Serum samples from the patients and the controls were isolated immediately after blood sampling of peripheral blood, stored at -20 C for 24 h, and then transferred to -50 C until the time of the assay. The protein concentration of the tissue was measured by the method of Lowry [10]. Superoxide dismutase (SOD) activity was measured according to the method described by Fridovich [11]. Lipid peroxidation level in the serum was expressed as MDA and was measured according to the procedure of Ohkawa et al. [12]. Nitric oxide (NO) levels were determined according to the Griess method [13]. The serum TAS levels were evaluated using a novel automated and colorimetric measurement method developed by Erel [8]. This is a 2,2 V-azinobis (3-ethylbenzo-thiazoline-6-sulfonate) (ABTSS?)-based method, in which a colorless molecule— reduced ABTS—is oxidized to a characteristic blue-green ABTSS?. When the colored ABTSS? is mixed with any substance that can be oxidized, it is reduced to its original colorless ABTS form again, where in contrast the reacted substance is oxidized. This feature is the basic principle of the methods that use the ABTS. The reduced ABTS molecule is oxidized to ABTSS? using hydrogen peroxide alone in acidic medium (an acetate buffer of 30 mmol/l pH 3.6). In the acetate buffer solution, the concentrated (deep green) ABTSS? molecules stay stable for a long time. When it is diluted with a more concentrated acetate buffer solution at higher pH-levels (an acetate buffer of 0.4 mol/l pH 5.8), the color is spontaneously and slowly bleached. Antioxidants present in the sample accelerate the bleaching rate to a degree proportional to their concentrations. This reaction can be monitored spectrophotometrically and the bleaching rate is inversely correlated with the TAS content of the sample. The reaction rate is calibrated with Trolox, which is widely used as a traditional standard for TAS measurement assays. The assay has excellent precision values which are lower than 3 %. The TAS levels are expressed as nmol Trolox Equiv./mg protein. The serum TOS levels were also evaluated using a novel automated and colorimetric measurement method developed by Erel [7]. Oxidants present in the sample oxidize Acta Neurol Belg 123 the ferrous ion–o-dianisidine complex to ferric ion (Reagent A: 5 mM ferrous ammonium sulfate and 10 mM o-dianisidine dihydrochloride). The oxidation reaction is enhanced by glycerol molecules, which are abundantly present in the reaction medium. The ferric ion makes a colored complex with xylenol orange in an acidic medium (Reagent B: 150 lM xylenol orange, 140 mM NaCl and 1.35 M glycerol). The color intensity that can be measured spectrophotometrically is related to the total amount of oxidant molecules present in the sample. The assay is calibrated with hydrogen peroxide, and the results are expressed in terms of nmol H2O2 Equiv./mg protein [14]. The serum OSI value was calculated as follows: OSI = [(TOS, lmol H2O2 Equiv./g protein)/(TAS, lmol H2O2 Equiv./g protein) 9 100] [9].
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS), version 11.5, for Windows (SPSS, Chicago, IL, USA). Data were expressed as mean ± standard deviation. The normality of the distribution for all variables was assessed by the Kolmogorov–Smirnov test. Student’s t test was used for normally distributed variables and Mann–Whitney U test was used for non-parametric variables. Relationships between variables were analyzed by Pearson or Spearman correlation analysis according to distribution type of parameters. A p\0.05 was considered to be statistically significant.
Results
The demographic characteristics of the MS patients and control subjects are given in Table 1.
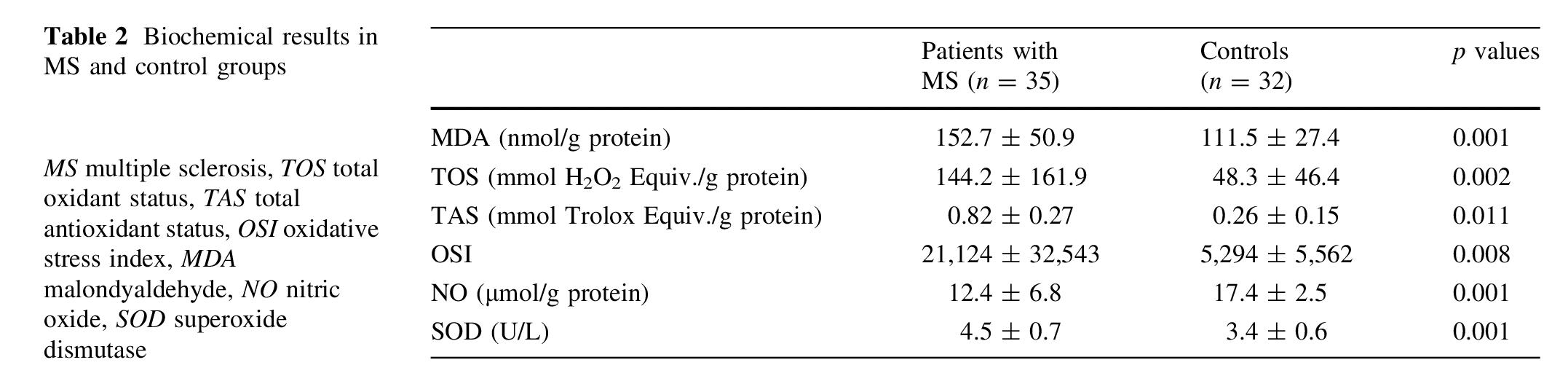
The oxidative/antioxidative data of the MS and control groups are shown in Table 2.
MS patients had higher concentrations of MDA (151.5 ± 51.1 vs. 111.3 ± 27.4 nmol/g protein, respectively; p\0.001), TOS (148.1 ± 162.5 vs. 48.3 ± 46.4 (mmol H2O2 Equiv./g protein, respectively; p = 0.002), OSI (21,124 ± 32,543 vs. 5,294 ± 5,562, respectively; p = 0.008), and SOD (4.5 ± 0.7 vs. 3.4 ± 0.6 U/L, respectively; p\0.001) compared with healthy controls. On the other hand, MS patients had lower concentrations of NO (12.3 ± 6.9 vs. 17.4 ± 2.5 lmol/g protein, respectively; p\0.001) and TAS (0.82 ± 0.27 vs. 0.26 ± 0.15, respectively; p = 0.011) compared with healthy controls. However, no correlation was observed between the oxidative stress markers (MDA, TAS, TOS, OSI, SOD and NO) and the clinic parameters of MS (number of attacks, EDSS score and duration of the disease) (p>0.05).
Discussion
In the present study, the elevation in the TOS, MDA, and OSI levels and the simultaneous decrease in the NO and TAS levels in the serum of MS patients provided evidence for an increased oxidative stress participating in the pathogenesis of the disease. Although the pathogenesis of MS has not been fully elucidated yet, it is believed that the inflammatory environment in demyelinating lesions lead to the generation of oxygen and nitrogen free radicals as well as proinflammatory cytokines, which in turn contribute to the development and progression of the disease [15]. The production of free oxygen radicals (e.g., hydroxyl, superoxide radicals) leads to oxidative stress when there is an imbalance in the redox status of a cell [16]. Various oxidative stress markers have been investigated in MS and the oxidative stress has been reported to be generally increased in MS patients [16, 17]. It has been demonstrated that the serum lipid peroxidation rates, plasma fluorescent lipid peroxidation products, the cholesteryl ester hydroperoxides which are a marker of the lipid peroxidation, thiobarbituric acid products, MDA and 4-hydroxy-alkenals, and conjugated dien levels are higher in MS patients [5, 17–21]. Previous studies have reported significant elevations of blood MDA levels in MS [19, 20, 22]. Similar to various previous studies, in our case we have also observed that the MDA levels, which are markers of lipid peroxidation, are increased in the serum of MS patients [19, 20, 22]. It should also be mentioned that there is only one study in the literature that measured the TOS levels in MS patients, which can be used to assess total oxidants [23]. Oxidative stress occurs due to the excessive production of oxidants, reduction of antioxidants, or a combination of both within the body. Kurban et al. have reported a statistically insignificant increase in the TOS level, a general marker of oxidant molecules, within the MS group in comparison with the control group. Besides, they have also demonstrated a significant decrease in the TAS molecules indicating the level of all the antioxidant markers in MS patients compared with the controls [23]. We have found in our study that the TOS levels in the serum of the MS patients were significantly higher and the TAS levels were significantly lower in comparison with the control group. This may occur due to the depletion of the endogenous antioxidants by the increased amounts of oxidants in MS. Also, the OSI has been studied for the first time in the serum of the MS patients in our study. During oxidative stress, sometimes only the antioxidants are depleted or only the oxidants are increased in the blood. Since OSI reveals the ratio of the total oxidants to the antioxidants, it demonstrates oxidative balance clearer than any other of the oxidative stress markers. We have shown in our study for the first time that together with the increase in the TOS levels and the decrease in the TAS levels, the OSI is also significantly increased in the serum of the MS patients. These findings may prove that the oxidative stress plays an important role in the pathogenesis of MS. In a large number of studies, various antioxidant molecules have been shown to decrease in MS [17, 24]. Besler et al. [17] have also demonstrated that the antioxidant vitamins (alpha tocopherol, beta-carotene, retinol and ascorbic acid) are decreased together with the antioxidant capacity in MS. Syburra and Passi [25], on the other hand, have shown that plasma levels of Vitamin E and ubiquinon, a liposoluble endogenous antioxidant, are significantly decreased in MS in comparison with the control group. In a recent study, it was found that uric acid levels were significantly lower when measured during a relapse than in a remission period in the patients with MS, as endogenous antioxidants [24]. Similarly, Choi et al. [26] reported that lower glutathione levels in patients with MS point to the presence of oxidative stress. Ghabaee et al. [27] have observed a decrease in antioxidant activity in the serum of patients with MS. The determination of serum TAS level reveals the body redox status better than the determination of the level of only one antioxidant in circulation [27]. Therefore, we chose to determine the TAS level indicating the whole antioxidant capacity in the serum of MS patients in our study. It seems that the low level of TAS observed in the serum of the patients may depend on the low levels of endogenous antioxidants such as uric acid. In line with the literature, we have also demonstrated that the TAS levels are decreased in the serum of MS patients. Besides the serum TAS levels, we have also studied the serum SOD activity in the MS patients. The first line of defense against oxidative stress is provided by SOD, a group of metal-containing anti-oxidant enzymes that catalyze the dismutation of superoxide anion to molecular oxygen and hydrogen peroxide [28]. Various observations suggest the involvement of SOD in neurodegeneration and neuroinflammation. Increased expression of SOD has been described for various oxidative stress-associated neurodegenerative and neuroinflammatory diseases [29]. In MS, significantly enhanced gene expression of SOD1 has been observed in active demyelinating lesions [30]. In our study, the SOD activity was significantly increased in the serum of the patients with MS. The increase in SOD under conditions of continuous oxidant production is likely to be a compensatory mechanism which protects the cells from further oxidant damage. Nitric oxide has a wide range of functions in both physiology and pathology. In vivo, NO is rapidly converted into its metabolites nitrate and nitrite, and the serum concentration of these metabolites can be used to estimate the NO levels. NO is supposed to play a role in the pathophysiology of MS. In active MS lesions, microglia and activated astrocytes express the enzyme-inducible NO synthase which enables them to produce large amounts of NO. NO is believed to contribute to the disease process of MS by direct tissue damage, blocking axonal conduction, and inducing axonal degeneration [31]. Although there is some controversy, the majority of studies relating to the involvement of NO in MS report higher NO activity in patients with MS compared with controls. In previous studies, higher levels of NO metabolites were found in the CSF of MS patients [32, 33]. De Bustos and colleagues did not find any difference in CSF nitrate concentrations between the control subjects and MS patients. Furthermore, serum nitrate concentrations were found to be lower than that in the healthy controls [34]. Ikeda et al. [35] found similar results about the CSF nitrate concentrations. In our study, similar to a previous study, the NO levels were significantly lower than that in the control group [35]. These contrasting results are likely to be related to different Table 2 Biochemical results in MS and control groups MS multiple sclerosis, TOS total oxidant status, TAS total antioxidant status, OSI oxidative stress index, MDA malondyaldehyde, NO nitric oxide, SOD superoxide dismutase Patients with MS (n = 35) Controls (n = 32) p values MDA (nmol/g protein) 152.7 ± 50.9 111.5 ± 27.4 0.001 TOS (mmol H2O2 Equiv./g protein) 144.2 ± 161.9 48.3 ± 46.4 0.002 TAS (mmol Trolox Equiv./g protein) 0.82 ± 0.27 0.26 ± 0.15 0.011 OSI 21,124 ± 32,543 5,294 ± 5,562 0.008 NO (lmol/g protein) 12.4 ± 6.8 17.4 ± 2.5 0.001 SOD (U/L) 4.5 ± 0.7 3.4 ± 0.6 0.001 Acta Neurol Belg 123 clinical conditions and/or disease severity of the patients. The apparent discrepancy may have various reasons including the diet of the patients, interaction with pharmacotherapy, or other methodological differences. In conclusion, it has been demonstrated in our study that the TAS and NO levels in the serum of the MS patients are lower and the serum TOS, MDA, OSI, and SOD levels are higher in comparison with the healthy individuals. Our findings confirm the presence of systemic oxidative stress in patients with MS. Whether antioxidant therapy in addition to immunomodulatory therapies may reduce disease activity in MS needs to be investigated in clinical trials.
Acknowledgment
This research was part of a project that was financially supported by Research Foundation of Dicle University, so we are thankful for this support.
References
1. Furlan R, Rovaris M, Martinelli Boneschi F, Khademi M, Bergami A, Gironi M, Deleidi M, Agosta F, Franciotta D, Scarpini E, Uccelli A, Zaffaroni M, Kurne A, Comi G, Olsson T, Filippi M, Martino G (2005) Immunological patterns identifying disease course and evolution in multiple sclerosis patients. J Neuroimmunol 165:192–200
2. Haider L, Fischer MT, Frischer JM, Bauer J, Ho¨ftberger R, Botond G, Esterbauer H, Binder CJ, Witztum JL, Lassmann H (2011) Oxidative damage in multiple sclerosis lesions. Brain 134:1914–1924
3. Miller E, Mrowicka M, Malinowska K, Mrowicki J, Saluk-Juszczak J, Ke˛dziora J (2011) Effects of whole-body cryotherapy on a total antioxidative status and activities of antioxidative enzymes in blood of depressive multiple sclerosis patients. World J Biol Psychiatry 12:223–227
4. Lutskii MA, Esaulenko IE (2007) Oxidant stress in the pathogenesis of multiple sclerosis. Neurosci Behav Physiol 37:209– 213
5. Ferretti G, Bacchetti T, Principi F, Di Ludovico F, Viti B, Angeleri VA, Danni M, Provinciali L (2005) Increased levels of lipid hydroperoxides in plasma of patients with multiple sclerosis: a relationship with paraoxonase activity. Mult Scler 11:677–682
6. Uzar E, Koyuncuoglu HR, Uz E, Yilmaz HR, Kutluhan S, Kilbas S, Gultekin F (2006) The activities of antioxidant enzymes and the level of malondialdehyde in cerebellum of rats subjected to methotrexate: protective effect of caffeic acid phenethyl ester. Mol Cell Biochem 291:63–68
7. Erel O (2005) A new automated colorimetric method for measuring total oxidant status. Clin Biochem 38:1103–1111
8. Erel O (2004) A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem 37:277–285
9. Alp R, Selek S, Alp SI, Tas¸kin A, Koc¸yig˘it A (2010) Oxidative and antioxidative balance in patients of migraine. Eur Rev Med Pharmacol Sci 14:877–882
10. Lowry OH, Rosebrough NJ, Farr AL, Randal RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
11. Fridovich I (1974) Superoxide dismutase. Adv Enzymol Relat Areas Mol Biol 41:35–97
12. Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
13. Cortas NK, Wakid NW (1990) Determination of inorganic nitrate in serum and urine by a kinetic cadmium-reduction method. Clin Chem 36:1440–1443
14. Hu ML, Louie S, Cross CE, Motchnik P, Halliwell B (1993) Antioxidant protection against hypochlorous acid in human plasma. J Lab Clin Med 121:257–262
15. Schreibelt G, van Horssen J, van Rossum S, Dijkstra CD, Drukarch B, de Vries HE (2007) Therapeutic potential and biological role of endogenous antioxidant enzymes in multiple sclerosis pathology. Brain Res Rev 56:322–330
16. Mirshafiey A, Mohsenzadegan M (2009) Antioxidant therapy in multiple sclerosis. Immunopharmacol Immunotoxicol 31:13–29
17. Besler HT, Comog˘lu S (2003) Lipoprotein oxidation, plasma total antioxidant capacity and homocysteine level in patients with multiple sclerosis. Nutr Neurosci 6:189–196
18. Koch M, Mostert J, Arutjunyan AV, Stepanov M, Teelken A, Heersema D, De Keyser J (2007) Plasma lipid peroxidation and progression of disability in multiple sclerosis. Eur J Neurol 14:529–533
19. Besler HT, Comog˘lu S, Okc¸u Z (2002) Serum levels of antioxidant vitamins and lipid peroxidation in multiple sclerosis. Nutr Neurosci 5:215–220
20. Ortiz GG, Macı´as-Islas MA, Pacheco-Moise´s FP, Cruz-Ramos JA, Sustersik S, Barba EA, Aguayo A (2009) Oxidative stress is increased in serum from Mexican patients with relapsing-remitting multiple sclerosis. Dis Markers 26:35–39
21. Koch M, Ramsaransing GS, Arutjunyan AV, Stepanov M, Teelken A, Heersema DJ, De Keyser J (2006) Oxidative stress in serum and peripheral blood leukocytes in patients with different disease courses of multiple sclerosis. J Neurol 253:483–487
22. Mitosek-Szewczyk K, Gordon-Krajcer W, Walendzik P, Stelmasiak Z (2010) Free radical peroxidation products in cerebrospinal fluid and serum of patients with multiple sclerosis after glucocorticoid therapy. Folia Neuropathol 48:116–122
23. Kurban S, Akpınar Z, Mehmetog˘lu I (2010) Investigation of serum paraoxonase and arylesterase activities and oxidative stress in patients with multiple sclerosis. Genel Tıp Derg (Turkish) 20:13–18
24. Guerrero AL, Gutie´rrez F, Iglesias F, Martı´n-Polo J, Merino S, Martı´n-Serradilla JI, Laherra´n E, Tejero MA (2011) Serum uric acid levels in multiple sclerosis patients inversely correlate with disability. Neurol Sci 32:347–350
25. Syburra C, Passi S (1999) Oxidative stress in patients with multiple sclerosis. Ukr Biokhim Zh 71:112–115
26. Choi IY, Lee SP, Denney DR, Lynch SG (2011) Lower levels of glutathione in the brains of secondary progressive multiple sclerosis patients measured by 1H magnetic resonance chemical shift imaging at 3 T. Mult Scler 17:289–296
27. Ghabaee M, Jabedari B, Al-E-Eshagh N, Ghaffarpour M, Asadi F (2010) Serum and cerebrospinal fluid antioxidant activity and lipid peroxidation in Guillain–Barre syndrome and multiple sclerosis patients. Int J Neurosci 120:301–304
28. Johnson F, Giulivi C (2005) Superoxide dismutases and their impact upon human health. Mol Aspects Med 26:340–352
29. Singh BK, Kumar A, Ahmad I, Kumar V, Patel DK, Jain SK, Singh C (2011) Oxidative stress in zinc-induced dopaminergic neurodegeneration: implications of superoxide dismutase and heme oxygenase-1. Free Radic Res 45:1207–1222
30. Tajouri L, Mellick AS, Ashton KJ, Tannenberg AE, Nagra RM, Tourtellotte WW, Griffiths LR (2003) Quantitative and qualitative changes in gene expression patterns characterize the activity of plaques in multiple sclerosis. Brain Res Mol Brain Res 119:170–183
31. Koch M, Mostert J, Arutjunyan A, Stepanov M, Teelken A, Heersema D, De Keyser J (2008) Peripheral blood leukocyte NO production and oxidative stress in multiple sclerosis. Mult Scler 14:159–165
32. Yamashita T, Ando Y, Obayashi K, Uchino M, Ando M (1997) Changes in nitrite and nitrate (NO2-/NO3-) levels in cerebrospinal fluid of patients with multiple sclerosis. J Neurol Sci 153:32–34
33. Brundin L, Morcos E, Olsson T, Wiklund NP, Andersson M (1999) Increased intrathecal nitric oxide formation in multiple sclerosis; cerebrospinal fluid nitrite as activity marker. Eur J Neurol 6:585–590
34. de Bustos F, Navarro JA, de Andre´s C, Molina JA, Jime´nezJime´nez FJ, Ortı´-Pareja M, Gasalla T, Tallo´n-Barranco A, Martı´nez-Salio A, Arenas J (1999) Cerebrospinal fluid nitrate levels in patients with multiple sclerosis. Eur Neurol 41:44–47
35. Ikeda M, Sato I, Matsunaga T, Takahashi M, Yuasa T, Murota S (1995) Cyclic guanosine monophosphate (cGMP), nitrite and nitrate in the cerebrospinal fluid in meningitis, multiple sclerosis and Guillain-Barre´ syndrome. Int Med 34:734–737